Some consider it controversial. Some consider it life changing. A new procedure, called transcranial magnetic stimulation, is proving effective for treating major depression. Neurostar is the first device approved by the U.S. Food and Drug Administration to perform transcranial magnetic stimulation, or TMS. With this device, the clinician uses magnets to send electrical currents to the frontal left side of the brain. The procedure activates neurotransmitters associated with the symptoms of depression: serotonin, norepinephrine and dopamine.
The treatment was first cleared by the FDA in October, 2008. Patients sit in a reclining chair with a coil placed against their scalp. The coil sends brief magnetic pulses to stimulate nerve cells in the brain. The procedure is done on an outpatient basis and typically administered for 40-minute sessions over four to six weeks. Dr. Mark George developed the treatment in the 1990s and recommends it for patients who have tried at least one antidepressant medication and failed to get better.
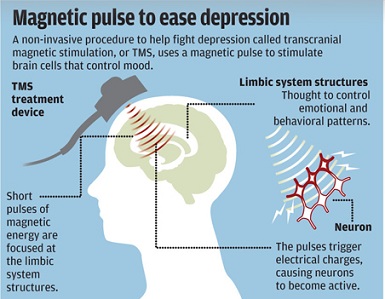
The theory behind the technology is that certain parts of the brain are less active in people who are depressed. Using the magnets can encourage brain cells in those areas to fire more often, regulating mood and depression. The idea is not that different from electroconvulsive therapy, a longstanding practice to treat depression that involves running a controlled electrical current through the brain. One of the benefits of TMS compared to electroconvulsive therapy is that is does not require anesthesia. In addition, there is a low rate of discontinuation due to side effects, which are most commonly headaches, and there are no side effects typically associated with antidepressant drugs. However, the procedure does have a risk of seizures, and there are no long-term studies of the effects.
Additionally, there are those who do not believe TMS should be used in a clinical setting. Public Citizen, a national consumer advocate watchdog group, has said that the medical device should not have been approved because its effectiveness was unproven. The group wrote a 2010 paper criticizing the federal approval process and saying that devices such as NeuroStar are not held to as stringent standards as pharmaceutical drugs.
One recently published study reflects the effectiveness of TMS. Among 307 patients across the country who received the treatment, doctors found that depression eased in 58 percent of them including 37 percent who reported that the depression was gone.
Skeptics, however, said that the study did not include a control group with patients receiving a treatment that did not deliver the electrical pulses. Many depression cases respond to a placebo effect, meaning depression often eases by itself or when people believe they are being treated for it. Studies that have included a control group showed smaller gains.
Another drawback is that insurers do not typically cover the procedure. Patients nationally are charged between $12,000 to $15,000 for the treatments. Still, the therapy has been performed at the Mayo Clinic in Minnesota since 2002. And it has also been featured on the Dr. Oz show. Despite the controversies, many patients are finding hope in a little magnet.
Lara Mossa Stump
and
Hulet Smith, OT